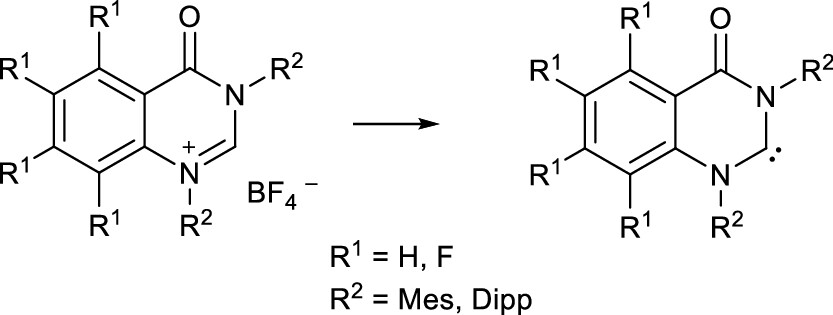
We present a new pathway toward cationic N,N′-diaryl-substituted quinazolin-4-one derivatives, which act as precursors for the synthesis of new N,N′-diaryl-amino-amido carbenes 5a,b that are stable under inert conditions. A tetrafluoro analogue 5c withstands isolation but can be prepared and employed in situ. The new NHCs exhibit a strong σ-donor capacity as well as strong π-accepting properties according to NMR and IR spectroscopic investigations indicated by the coupling constants 1JC,H (210–214 Hz) and 77Se NMR resonances (545–654 ppm) of suitable derivatives. Remarkably, replacement of the hydrogen atoms of the anellated benzene ring by fluorine atoms exerts only a small effect on the overall donor properties of the carbene, as suggested by a small shift of the TEP values from 2053 cm–1 (5a) to 2055 cm–1 (5c). An ambiphilic reactivity of the NHCs 5 is supported by their reactions with isonitriles to provide the respective ketenimines 13, which show unprecedented rearrangement reactions, induced by either water or heat. The NHCs 5 also react with amines or methanol in N–H and O–H bond activations. The mechanisms of these activation reactions were investigated by quantum chemical calculations. Due to the apparent blue emission of the selected derivatives, the photophysical properties were exemplarily characterized in solution and solid state and assigned to the underlying electronic transitions by (TD)-DFT calculations.
Read more in:
Stable N-Heterocyclic Carbenes with the N,N′-Diarylquinazolin-4-one Backbone: Improved Synthesis, Electronic Properties, and Reactivity
J. Maurice Pütz, Simone T. Hauer, Joscha Nellesen, Daniel Deißenbeck, Thomas J. J. Müller, Jan Meisner, Christian Ganter
Organometallics, 43, 2, 141-163, 2024